UCL Ionic liquids research Project

Young people from secondary schools and colleges across the UK gathered this June to share their scientific research with peers and the wider secondary community at the IRIS Student Conferences.
The Institute for Research in Schools (IRIS) aims to change the culture in UK education to make authentic research part of every young person’s experience and more than 1,500 students have taken part in their projects this year.
IRIS runs a range of projects. We chose to participate in the ionic liquids project. Ionic substances are very common and the most ubiquitous example is probably sodium chloride - table salt. These substances are usually crystalline solids at room temperature with high melting points. Table salt melts at about 800 ℃. However, it is possible to synthesise ionic substances that have much weaker bonding and this allows them to be liquids at room temperature. We call them Room Temperature Ionic Liquids (RTILs).
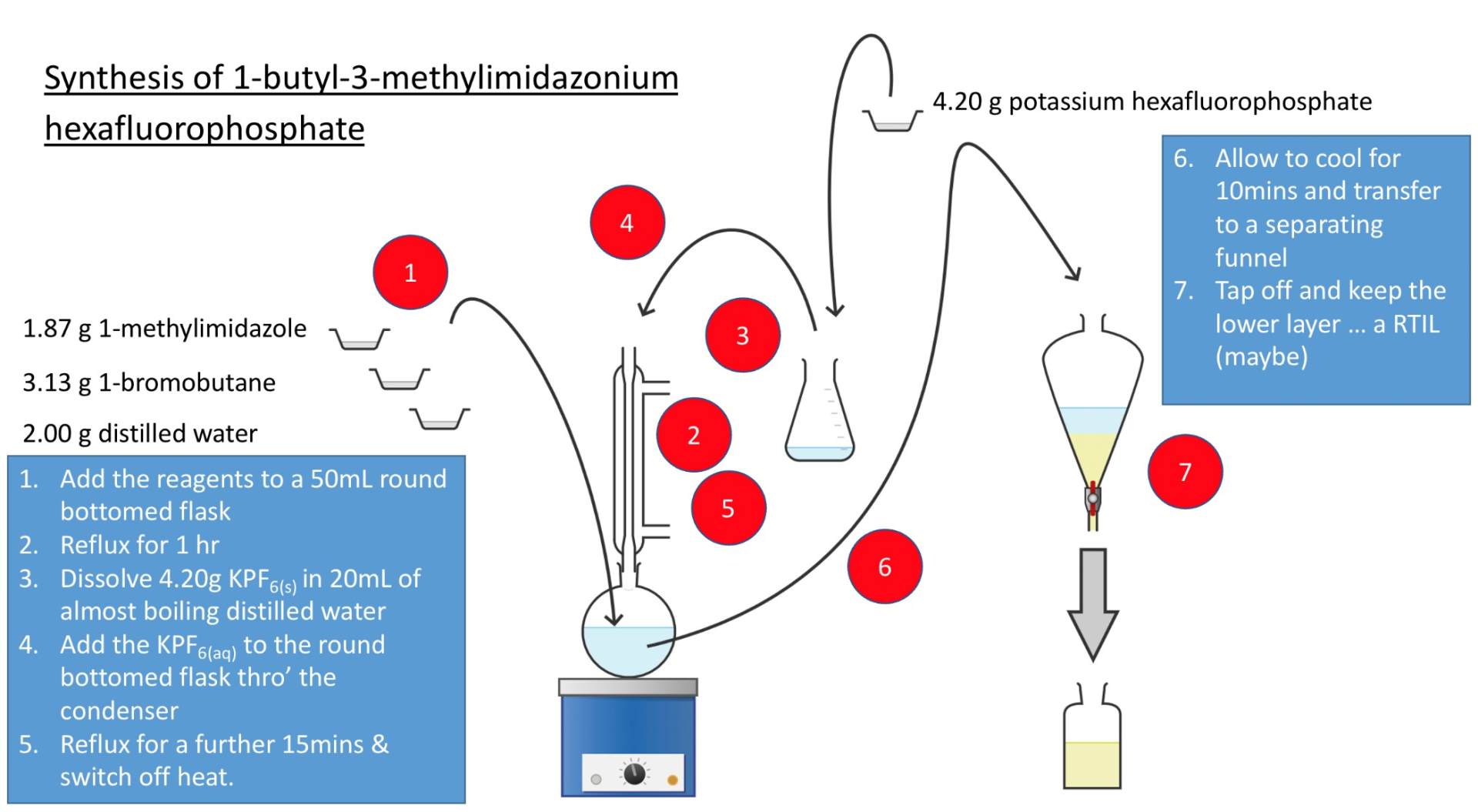

Our students have synthesised RTILs and have planned a next generation of RTILs which they aim to characterise with the assistance of the Department of Chemistry at UCL. The work done by Graveney students Emily, Khadidja, Ben, Raiyan and Khalid is summarised in their poster:
RTILs are interesting because of their properties, for example:
- They conduct electricity very efficiently and do not evaporate which makes them attractive as components in new generation vehicle batteries because they would be safe in the event of a crash;
- They are potent solvents and have potential to be used to extract valuable materials in a greener way than current technologies allow;
- They are “hydrotropic” agents which means that they increase the solubility of other substances. This has potential in the pharmaceutical industry because many pharmaceutical agents are rather insoluble. This means they have poor bioavailability. If the solubility of these agents can be increased, their efficacy would be enhanced.